When you picture a product manager, images of tech companies or cutting-edge software might come to mind. However, this pivotal role exists in the pharmaceutical industry too, albeit with unique challenges and responsibilities. Unlike their tech counterparts, pharma product managers oversee life-saving therapies, navigating complex regulatory landscapes and lengthy product lifecycles.
The global pharmaceutical market was estimated at USD 1,559 billion in 2023 and is expected to surpass around USD 2,832 billion by 2033, according to vision research reports. Within this vast industry, product managers play a critical role in bringing new drugs to market and ensuring their success.
Unlike tech products, pharmaceutical products often take a decade or more to develop, with only 1 in 10,000 compounds making it from the lab to the market. This lengthy and high-stakes process makes the role of a pharmaceutical product manager all the more critical. They must possess a deep understanding of the science behind their products, the regulatory environment, and the commercial landscape to ensure that their therapies reach the patients who need them.
The pharma product management journey could begin years before a drug gets approval and can continue long after. Their responsibilities evolve with each phase of the product lifecycle.
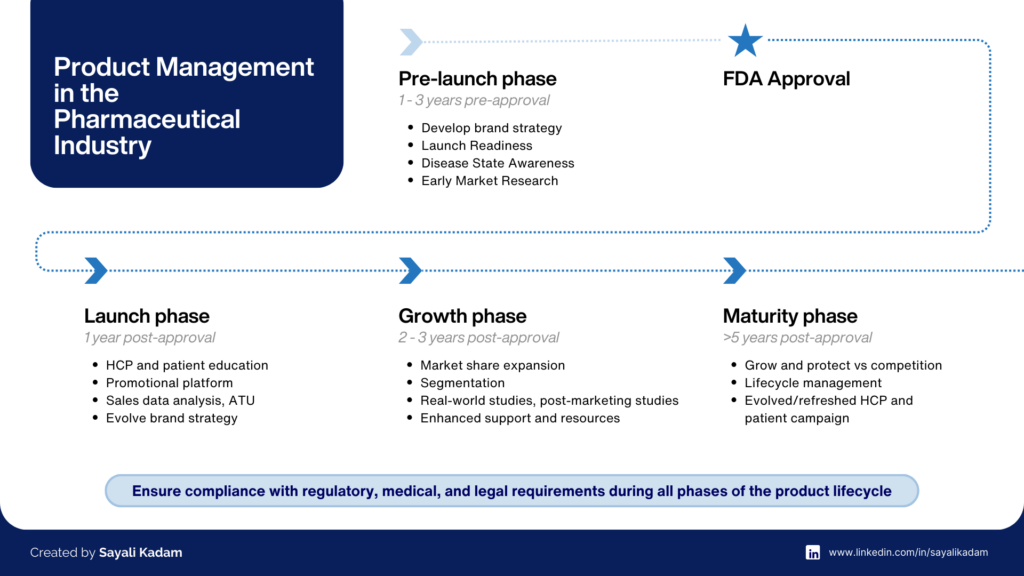
In this phase, a product manager could be appointed anywhere from three to one year prior to the anticipated FDA approval. This period is critical for setting the foundation of the product's success, while the drug is still in clinical trials with many unknowns. The ability to work effectively in this "white space" is crucial.
Some of the primary objectives of the product manager are to identify market needs through extensive market research, conducting competitive analysis, and forecasting future demand. A key deliverable is drafting the product's brand strategy and positioning – the foundation for how it will be perceived by healthcare professionals and patients. This strategy is crucial, as data has shown that two-thirds of pharmaceutical product launches don’t meet performance expectations.
Product managers also initiate pre-approval market readiness, laying the groundwork for a successful launch. This includes developing a robust scientific platform, forming the creative brand identity, engaging Key Opinion Leaders (KOLs), and planning market access strategy.
This pre-launch period is one of the most exciting times to be on the team, as it's when the brand strategy and creative direction are set. However, it's also challenging, requiring product managers to navigate uncertainties while making critical decisions that will shape the product's future in the market.
The first year of a product’s launch is the most important time, as it is a strong predictor of future year’s performance. This period sets the foundation for long-term market performance and patient adoption.
To successfully navigate the launch phase, product managers will focus on empowering the sales force through comprehensive training and ongoing support. Simultaneously, they will continue engaging KOLs, leveraging their influence to drive awareness and adoption among peers. HCP education will be a priority, utilizing a variety of materials and events to effectively communicate product benefits and appropriate patient selection. Patient education and support will also be emphasized, ensuring patients have access to user-friendly resources and assistance programs. Building relationships with payers and formulary decision-makers will be crucial, presenting compelling clinical and economic evidence to secure favorable formulary placement and reimbursement. Finally, continuous market monitoring and feedback mechanisms will be established to track key performance indicators, gather insights, and adapt marketing strategies accordingly, ensuring a successful and impactful launch of the new therapy.
The growth phase is a critical phase for the brand, marking it a period of intensified efforts to capitalize on the initial launch momentum and propel the product towards peak sales. This phase demands a strategic shift in the product manager's role, transitioning from launch execution to a focus on expansion and optimization.
During this pivotal time, product managers are tasked with driving significant market share expansion, leveraging data-driven insights to identify and target untapped segments. Furthermore, product managers will actively explore new indications for the product, capitalizing on emerging scientific evidence and unmet medical needs.
This is also a time when real-world evidence and post-marketing studies can be leveraged to reinforce the product’s value proposition. Additionally, product managers can prioritize improving the customer experience by gathering feedback from patients, healthcare providers, and payers to understand their evolving needs and preferences. This customer-centric approach can lead to the development of enhanced patient support programs, educational resources, and innovative delivery mechanisms that cater to the specific requirements of different stakeholders. By navigating this critical period with a strategic approach, product managers can position the brand for continued success and sustained growth in the years to come.
As the product matures, the product team needs to focus on not only growing the brand but also protecting its market share. They have to defend against competitors, explore innovative ways to keep the product relevant and prepare for the eventual entry of generic rivals. Strengthen relationships with key stakeholders, including healthcare providers, payers, and patient advocacy groups, to reinforce brand loyalty and advocacy. Marketing messages need to evolve to reflect new competitive positioning.
Llifecycle management optimization is paramount during this phase. Product managers must proactively assess the product's lifecycle, anticipate potential threats such as patent expiration or generic competition, and develop strategies to mitigate these risks. This often involves exploring new formulations, dosage forms, or combination therapies.
By focusing on these key strategies, product managers can effectively navigate the maturity phase, defending market share, exploring growth opportunities, and preparing for the future, ultimately ensuring the continued success and relevance of the brand in an ever-evolving healthcare landscape.
Pre-Launch: My first foray into pharmaceutical product management began a year before the expected FDA approval of a novel therapy. There were many unknowns as Phase III trials were ongoing, but I researched the market, analyzed the competitive landscape, and developed a brand strategy for the product. I partnered with my clinical and medical colleagues to create a Target Product Profile (TPP) that served as the foundation for market research for developing brand strategy and creative campaign. Robust scenario planning was critical to ensure we were ready for multiple label language scenarios and product supply availability. I also laid the foundation for a robust scientific strategy with congress engagement, speaker program, and KOL outreach.
Launch: I'll never forget the Friday afternoon when we received the long-awaited FDA approval. Weeks of meticulous planning cross-functionally had culminated in a day-by-day plan for the first 15 days post-approval. It included a robust communication plan, website update, PI national call and certification for field, call center readiness, and ensuring that there was a plan for all marketing assets to be ready for deployment as soon as possible. A unique thing working in this industry is that all externally facing assets have to be submitted to FDA for review. This added regulatory and compliance requirements of the industry necessitate extensive planning and coordination.
Another key activity in this critical launch year that I put together was a framework in place for receiving rapid updates and feedback from physicians, patients, and sales force, to evolve brand strategy or messaging as needed quickly. This included feedback collected via surveys, advisory boards, market research, and social listening reports.
Growth: During this phase, we faced several challenges that we had to overcome for product adoption and expansion. I worked closely with sales leadership to gain real-time feedback from the customers on barriers to adoption, and deployed several campaigns, tactics, and field direction to overcome these barriers. I also leveraged real-world evidence - both clinical and economic - to solidify the reasons to believe for product use. In addition, I monitored competitor activity and developed a competitive readiness and response plan.
Maturity: As the product matured and competition intensified, I had to think creatively to differentiate our brand and deepen our connection with customers. We shared compelling real-patient stories, launched mini-campaigns highlighting case studies and treatment guideline updates, and compiled a centralized resource of real-world publications. Additionally, I collaborated with our medical team to explore potential reformulations that could enhance patient convenience and further distinguish our product in the market.
This journey, marked by challenges, triumphs, and constant adaptation, exemplifies the dynamic role of a pharmaceutical product manager.
Pharmaceutical product managers must possess a diverse skill set to succeed:
In conclusion, unlike their counterparts in the tech industry, pharma product managers are not merely launching the latest gadget, app, or feature; they are bringing to market life-saving therapies that have the power to alleviate suffering, improve quality of life, and potentially extend life expectancy. They are the bridge between scientific innovation and patient care, ensuring that the remarkable discoveries made in labs translate into tangible benefits for millions around the globe.
Pharmaceutical product management is a challenging yet rewarding field. It's a career that offers intellectual stimulation, meaningful work, and the chance to make a lasting difference in the lives of countless individuals. Whether you're launching a groundbreaking new therapy, expanding its reach to new markets, or safeguarding its position in a competitive landscape, your work as a pharmaceutical product manager will be instrumental in advancing medical science and improving patient care.
Ultimately, the journey of a pharmaceutical product manager is one of continuous learning, adaptation, and growth. It's a career that demands resilience, creativity, and a deep understanding of the complex interplay between science, business,and healthcare. But for those who embrace the challenges and embrace the opportunities, the rewards are immeasurable.








Comments
Join the community
Sign up for free to share your thoughts